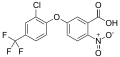
Acifluorfen
A herbicide used in agriculture
| Chemical Compound | |
|---|---|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider ID | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Properties | |
| Chemical Formula | |
| Molar Mass | |
| Appearance | |
| Density | |
| Melting Point | |
| Boiling Point | |
| Hazards | |
| GHS Pictograms | [[File:|50px]] |
| GHS Signal Word | |
| GHS Hazard Statements | |
| NFPA 704 | [[File:|50px]] |
| References | |
Acifluorfen is a herbicide used primarily in agriculture to control broadleaf weeds and grasses. It is a member of the diphenyl ether class of herbicides and is known for its effectiveness in post-emergence weed control.
Chemical properties
Acifluorfen is a diphenyl ether derivative with the chemical formula C14H7ClF3NO5. It is characterized by its ability to inhibit the protoporphyrinogen oxidase enzyme, which is crucial in the chlorophyll biosynthesis pathway. This inhibition leads to the accumulation of protoporphyrin IX, causing cell membrane disruption and ultimately plant death.
Mode of action
Acifluorfen acts by inhibiting the enzyme protoporphyrinogen oxidase (PPO), which is involved in the biosynthesis of chlorophyll. The inhibition of PPO leads to the accumulation of protoporphyrin IX, a photodynamic compound that, in the presence of light, generates reactive oxygen species. These reactive oxygen species cause lipid peroxidation, leading to the destruction of cell membranes and plant tissues.
Applications
Acifluorfen is used in various crops such as soybeans, peanuts, and rice. It is applied as a post-emergence herbicide, meaning it is used after the weeds have emerged from the soil. Its effectiveness is enhanced by the presence of sunlight, which activates the photodynamic process leading to weed control.
Safety and environmental impact
While acifluorfen is effective in controlling weeds, it is important to consider its environmental impact and safety profile. It is classified as a restricted use pesticide in some regions due to its potential to cause harm to non-target plants and aquatic organisms. Proper handling and application techniques are essential to minimize its impact on the environment.
Synthesis
The synthesis of acifluorfen involves several chemical reactions, starting from basic organic compounds. The process typically includes the formation of the diphenyl ether backbone, followed by the introduction of the nitro, chloro, and trifluoromethyl groups. The detailed synthesis pathway is illustrated in the accompanying diagram.
Related pages
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD