Buchwald–Hartwig amination
A chemical reaction used in organic synthesis
The Buchwald–Hartwig amination is a chemical reaction that forms carbon-nitrogen bonds by coupling an amine with an aryl halide or pseudohalide in the presence of a palladium catalyst. This reaction is a powerful tool in organic chemistry for the synthesis of amines, which are important in the production of pharmaceuticals, agrochemicals, and materials.
History
The reaction is named after Stephen L. Buchwald and John F. Hartwig, who independently developed the methodology in the 1990s. Their work built upon earlier studies of palladium-catalyzed cross-coupling reactions, such as the Suzuki reaction and the Stille reaction.
Mechanism
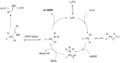
The Buchwald–Hartwig amination proceeds through a catalytic cycle involving several key steps:
- Oxidative Addition: The palladium(0) catalyst undergoes oxidative addition with the aryl halide to form a palladium(II) complex.
- Ligand Exchange: The amine displaces a ligand on the palladium complex, forming a palladium-amine complex.
- Reductive Elimination: The palladium complex undergoes reductive elimination to form the desired carbon-nitrogen bond, regenerating the palladium(0) catalyst.
Catalysts and Ligands
The choice of catalyst and ligand is crucial for the success of the Buchwald–Hartwig amination. Palladium catalysts such as palladium acetate or palladium chloride are commonly used. The ligands, often bulky phosphines, stabilize the palladium center and enhance the reaction's efficiency. Examples of ligands include Xantphos, BINAP, and SPhos.
Applications
The Buchwald–Hartwig amination is widely used in the synthesis of pharmaceuticals, where the formation of carbon-nitrogen bonds is essential. It is also employed in the production of natural products, polymers, and dyes. The reaction's versatility and efficiency make it a valuable tool in both academic and industrial settings.
Advantages and Limitations
The Buchwald–Hartwig amination offers several advantages, including high selectivity, mild reaction conditions, and broad substrate scope. However, it also has limitations, such as the need for expensive palladium catalysts and the potential for catalyst deactivation.
Gallery
Related pages
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD