Carbon suboxide
Carbon suboxide, or C3O2, is an oxide of carbon that is a colorless gas with a sharp odor. It is interesting for its unusual structure and for being one of the simplest molecules that exhibits polymerization. Carbon suboxide is also notable for its role in the chemistry of the atmosphere of Mars, as well as for its use in organic synthesis.
Structure and Properties
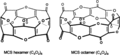
Carbon suboxide is composed of three carbon atoms and two oxygen atoms, with the molecular formula C3O2. The structure of carbon suboxide can be described as a linear chain of three carbon atoms with two terminal oxygen atoms. This molecule is a cumulene, meaning it contains a series of double bonds that alternate with single bonds. Specifically, its structure can be represented as O=C=C=C=O, which shows the cumulenic nature of the molecule.
The molecule is of interest due to its electronic structure and its ability to polymerize into larger networks. Carbon suboxide's polymerization can lead to the formation of various types of polymers, which have potential applications in materials science.
Synthesis
Carbon suboxide can be synthesized through the dehydration of malonic acid (C3H4O4) with phosphorus pentoxide (P2O5) acting as the dehydrating agent. The reaction proceeds through the removal of water (H2O) from malonic acid, leading to the formation of C3O2.
Reactions and Uses
Despite its instability, carbon suboxide has been studied for its reactivity and potential uses in organic synthesis. It can act as a building block for the synthesis of more complex organic compounds. Additionally, carbon suboxide has been investigated for its role in the atmosphere of Mars, where it could participate in chemical reactions that contribute to the planet's climate and potential for supporting life.
Safety and Precautions
Carbon suboxide is a reactive gas and should be handled with care in a controlled environment. It can pose risks due to its reactivity and the potential for polymerization under certain conditions.
In Popular Culture
While not widely known in popular culture, carbon suboxide's unique properties and potential applications in science and technology make it a subject of interest among chemists and researchers.
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD