Cyclopropanation
Cyclopropanation refers to a chemical reaction that introduces a cyclopropane ring into molecules. This process is significant in organic chemistry due to the unique properties and reactivity of cyclopropane rings, which are three-membered carbon rings. Cyclopropanation reactions are widely used in the synthesis of pharmaceuticals, agrochemicals, and other organic compounds.
Mechanism
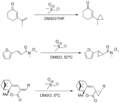
The cyclopropanation reaction typically involves the interaction between an alkene and a carbene or a carbenoid to form a cyclopropane ring. Carbenes are highly reactive species with a divalent carbon atom that has two non-bonded electrons. Carbenoids are compounds that behave similarly to carbenes in reactions but are not true carbenes.
Carbene Generation
Carbenes can be generated in situ through several methods:
- Decomposition of diazo compounds in the presence of a metal catalyst, such as copper or rhodium.
- Photolysis or thermolysis of precursors like diazirines or diazoalkanes.
- Dehalogenation of dihalocarbenes with zinc or silver.
Cyclopropanation Reaction
Once generated, the carbene or carbenoid species reacts with the double bond of an alkene, leading to the formation of a cyclopropane ring. This reaction can proceed through a concerted mechanism, where the carbene inserts into the C=C bond directly, or through a stepwise process involving free radical intermediates.
Types of Cyclopropanation Reactions
Several methods have been developed for cyclopropanation, including:
- The Simmons-Smith reaction, which uses a carbenoid generated from diiodomethane and a zinc-copper couple.
- The use of diazo compounds in the presence of a transition metal catalyst, a method known as metal-catalyzed cyclopropanation.
- The use of stable carbenes, such as N-heterocyclic carbenes, for cyclopropanation without the need for metal catalysts.
Applications
Cyclopropanation reactions are crucial in the synthesis of various biologically active compounds and natural products. The cyclopropane ring is a common motif in many pharmaceuticals due to its conformational rigidity, which can enhance the specificity and potency of drug molecules. Additionally, cyclopropanes are used in the synthesis of agrochemicals and materials science.
Safety and Environmental Considerations
The use of hazardous chemicals like diazo compounds and heavy metals in cyclopropanation reactions necessitates careful handling and disposal practices. Research is ongoing to develop more environmentally friendly and sustainable methods for cyclopropanation, including the use of less toxic reagents and catalysts.
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD