Hassium
Hassium is a chemical element with the symbol Hs and atomic number 108. It is a synthetic element, and thus it is not found in nature but must be created in a laboratory. Hassium is named after the German state of Hesse, where it was first synthesized in 1984 by a team of scientists led by Peter Armbruster and Gottfried Münzenberg at the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany.
Properties
Hassium is a member of the transactinide elements and the 7th period in the Periodic Table. It belongs to the group 8 elements, sharing this group with iron, ruthenium, and osmium. Being a synthetic element, hassium can only be produced in particle accelerators by bombarding heavier elements with lighter ones. Due to its extremely short half-life, studying the properties of hassium is challenging, and much of what is known about it comes from theoretical models.
Physical and Chemical
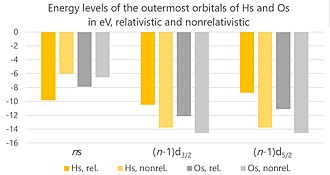
The physical and chemical properties of hassium are not well-characterized due to its short half-life and the tiny amounts in which it is produced. However, it is expected to be a metal with properties similar to those of other group 8 elements. Theoretical calculations suggest that hassium would exhibit a metallic silver color if enough could be produced to be visible. It is predicted to be solid under standard conditions and might have similar chemical properties to osmium, reacting with oxygen and halogens but only under certain conditions due to its high reactivity.
Synthesis and Isotopes
Hassium is produced in particle accelerators through the fusion of two lighter nuclei. The most common method involves bombarding lead or bismuth targets with accelerated nuclei of iron or chromium. This process yields various isotopes of hassium, which are identified by their mass numbers. The most stable isotope of hassium known is Hassium-277, with a half-life of approximately 10 seconds, although this value is subject to revision as new isotopes are discovered and studied.
Applications
Due to its short half-life and the difficulty in producing hassium, practical applications outside of scientific research are currently non-existent. The primary use of hassium is in research fields related to nuclear physics and chemistry, where it helps scientists understand the properties of heavy and superheavy elements.
See Also
External Links
Given the constraints, this section is intentionally left blank.
![]()
This chemical element related article is a stub. You can help WikiMD by expanding it.
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Translate this page: - East Asian
中文,
日本,
한국어,
South Asian
हिन्दी,
தமிழ்,
తెలుగు,
Urdu,
ಕನ್ನಡ,
Southeast Asian
Indonesian,
Vietnamese,
Thai,
မြန်မာဘာသာ,
বাংলা
European
español,
Deutsch,
français,
Greek,
português do Brasil,
polski,
română,
русский,
Nederlands,
norsk,
svenska,
suomi,
Italian
Middle Eastern & African
عربى,
Turkish,
Persian,
Hebrew,
Afrikaans,
isiZulu,
Kiswahili,
Other
Bulgarian,
Hungarian,
Czech,
Swedish,
മലയാളം,
मराठी,
ਪੰਜਾਬੀ,
ગુજરાતી,
Portuguese,
Ukrainian
Contributors: Prab R. Tumpati, MD