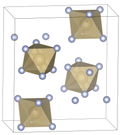
Osmium hexafluoride
Osmium hexafluoride (OsF6) is a chemical compound of osmium and fluorine. It is one of the few binary hexafluorides, a class of compounds where six fluorine atoms are bonded to a central metal atom. Osmium hexafluoride is notable for its role in the study of chemical bonding and molecular geometry, as well as its applications in various chemical processes.
Properties
Osmium hexafluoride is a volatile, crystalline solid at room temperature. It has a high melting point and sublimes at temperatures slightly above room temperature, making it a challenging substance to work with. The compound is highly reactive, especially with water, releasing toxic gases. It is one of the most potent oxidizing agents known and must be handled with extreme care in a controlled environment.
The molecular geometry of OsF6 is octahedral, a common shape for compounds with six ligands surrounding a central atom. This geometry is significant in the field of coordination chemistry and provides insights into the electronic structure of the osmium atom when it is in a high oxidation state.
Synthesis
Osmium hexafluoride is synthesized through the direct reaction of osmium metal with fluorine gas. This process requires careful control of reaction conditions, including temperature and pressure, to ensure the safety and yield of the product. The reaction is highly exothermic, releasing a significant amount of energy.
Applications
While osmium hexafluoride itself has limited applications due to its reactivity and toxicity, its study has contributed to the broader understanding of chemical bonding, molecular geometry, and the behavior of hexafluorides in general. Its properties are of interest in the development of new materials and in the field of inorganic chemistry.
Safety
Due to its high reactivity and toxicity, osmium hexafluoride requires stringent safety measures during handling and storage. It can cause severe burns upon contact with skin and is dangerous if inhaled. Laboratories working with OsF6 must be equipped with appropriate safety equipment, including fume hoods and protective clothing.
See also
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD