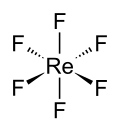
Rhenium hexafluoride
Rhenium hexafluoride is a chemical compound with the formula ReF6. It is one of the seventeen known binary hexafluorides. Rhenium hexafluoride is a dense, volatile, and highly reactive compound that is a colorless gas at room temperature, making it one of the most reactive and volatile compounds of rhenium. It is used in various chemical research applications and in the synthesis of other rhenium compounds.
Properties
Rhenium hexafluoride is a powerful oxidizing agent that can react violently with organic materials and is hydrolyzed by water to form oxygen and rhenium oxides. It has a high melting point and boils at temperatures significantly higher than room temperature, which makes it a gas under normal conditions. The compound has a complex electronic structure, with the rhenium atom exhibiting a +6 oxidation state, which is common for rhenium in its high oxidation state compounds.
Synthesis
The synthesis of rhenium hexafluoride typically involves the direct combination of rhenium with fluorine gas at high temperatures. This process requires careful control of reaction conditions to prevent the formation of unwanted byproducts and to ensure the purity of the ReF6 produced. The reaction is highly exothermic and must be conducted in apparatus made of materials that can withstand the corrosive effects of both reactants and the product.
Applications
Due to its potent oxidizing properties and volatility, rhenium hexafluoride is primarily used in scientific research. It serves as a fluorinating agent in the synthesis of other chemical compounds and can be used to introduce fluorine atoms into various molecular structures. Its applications are mainly confined to the laboratory due to its reactivity and the difficulty in handling the compound safely.
Safety
Handling rhenium hexafluoride requires strict safety precautions due to its high reactivity and toxicity. It can cause severe burns upon contact with skin and can be fatal if inhaled. Appropriate protective equipment, including gloves, goggles, and face shields, must be worn when working with this compound. Additionally, operations involving ReF6 should be conducted in a well-ventilated area or under a fume hood to avoid inhalation of fumes.
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD