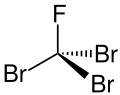
Tribromofluoromethane
A halomethane compound with the formula CBr_F
| Chemical Compound | |
|---|---|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider ID | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Properties | |
| Chemical Formula | |
| Molar Mass | |
| Appearance | |
| Density | |
| Melting Point | |
| Boiling Point | |
| Hazards | |
| GHS Pictograms | [[File:|50px]] |
| GHS Signal Word | |
| GHS Hazard Statements | |
| NFPA 704 | [[File:|50px]] |
| References | |
Tribromofluoromethane, also known as fluorotribromomethane, is a halomethane compound with the chemical formula CBr_F. It is a colorless liquid at room temperature and is primarily used in fire extinguishing systems.
Properties
Tribromofluoromethane is characterized by its high density and low boiling point. It is a non-flammable compound, which makes it suitable for use in fire suppression. The compound is stable under normal conditions but can decompose when exposed to high temperatures, releasing toxic gases such as hydrogen bromide and hydrogen fluoride.
Synthesis
Tribromofluoromethane can be synthesized through the halogenation of methane using bromine and fluorine. The process involves the substitution of hydrogen atoms in methane with bromine and fluorine atoms, resulting in the formation of CBr_F.
Applications
The primary application of tribromofluoromethane is in fire extinguishing systems, particularly in environments where water-based extinguishers are unsuitable. It is used in Halon fire suppression systems, which are effective in extinguishing fires without leaving residue. However, due to environmental concerns, the use of halons, including tribromofluoromethane, has been restricted under the Montreal Protocol due to their ozone-depleting potential.
Environmental Impact
Tribromofluoromethane is classified as an ozone-depleting substance. Its release into the atmosphere contributes to the depletion of the ozone layer, which protects the Earth from harmful ultraviolet radiation. As a result, its production and use have been phased out in many countries in favor of more environmentally friendly alternatives.
Safety
Handling tribromofluoromethane requires caution due to its potential to release toxic gases upon decomposition. Proper ventilation and protective equipment are recommended when working with this compound. In case of exposure, immediate medical attention is advised.
Related pages
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD