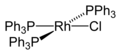
Wilkinson's catalyst
Wilkinson's catalyst, chemically known as chloridotris(triphenylphosphine)rhodium(I), is a coordination compound with the formula RhCl(PPh3)3. It is a homogeneous catalyst used in the hydrogenation of alkenes. The catalyst was first synthesized by the chemist Geoffrey Wilkinson, a Nobel Laureate, and its development marked a significant advancement in organometallic chemistry and homogeneous catalysis.
Structure and Properties
Wilkinson's catalyst is a square planar complex consisting of a rhodium center coordinated to three triphenylphosphine (PPh3) ligands and one chloride ion. This configuration provides the catalyst with its characteristic reactivity and selectivity in chemical reactions. The compound is soluble in organic solvents such as benzene, chloroform, and ethanol, which facilitates its use in homogeneous catalysis.
Synthesis
The synthesis of Wilkinson's catalyst involves the reaction of rhodium(III) chloride with an excess of triphenylphosphine in a boiling alcohol solution. The reaction proceeds via the reduction of rhodium(III) to rhodium(I) and the formation of the complex:
RhCl3·3H2O + 3 PPh3 → RhCl(PPh3)3 + 3 H2O + 2 HCl
Mechanism of Action
In the hydrogenation of alkenes, Wilkinson's catalyst facilitates the addition of hydrogen (H2) across the carbon-carbon double bond. The mechanism involves oxidative addition of H2 to the rhodium(I) center to form a rhodium(III) dihydride species. This is followed by coordination of the alkene to the metal center, insertion of the alkene into one of the Rh-H bonds (hydrometalation), and reductive elimination of the alkane from the metal. The cycle is completed when the catalyst is regenerated.
Applications
Wilkinson's catalyst is primarily used in the hydrogenation of alkenes to alkanes. It exhibits high selectivity, allowing for the hydrogenation of terminal alkenes in the presence of internal alkenes. Additionally, it has been employed in the hydrogenation of other unsaturated compounds, including aldehydes, ketones, and nitriles. Its ability to selectively hydrogenate less reactive bonds under mild conditions makes it a valuable tool in organic synthesis.
Safety and Environmental Considerations
As with many organometallic compounds, Wilkinson's catalyst should be handled with care. It is sensitive to air and moisture, requiring storage under an inert atmosphere. Proper safety equipment, such as gloves and eye protection, should be used when handling the compound. Disposal should follow regulations for hazardous waste, considering its potential environmental impact.
See Also
References
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD