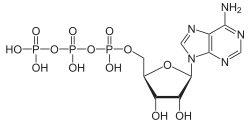
Adenosine triphosphate
Adenosine triphosphate (ATP) is a complex organic chemical that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer.
Structure
ATP consists of an adenosine molecule bonded to three phosphate groups. The adenosine molecule is composed of an adenine ring and a ribose sugar. The three phosphate groups are labeled alpha, beta, and gamma, starting with the group closest to the ribose. The bonds between these phosphate groups are high-energy bonds, which release energy when broken.
Function
ATP is used by cells as a coenzyme in various biochemical reactions. It is involved in metabolism, serving as a substrate for enzymes and signal transduction pathways. ATP is also crucial in DNA and RNA synthesis, where it acts as a building block.
Energy Transfer
The energy stored in ATP is released when it is hydrolyzed to adenosine diphosphate (ADP) and an inorganic phosphate. This reaction releases energy that can be used by the cell to perform work. The conversion of ATP to ADP is a reversible process, allowing ATP to be regenerated from ADP and phosphate through cellular respiration.
Role in Cellular Processes
ATP is essential for many cellular processes, including:
- Muscle contraction: ATP binds to myosin, allowing it to interact with actin filaments and produce muscle contraction.
- Active transport: ATP provides energy for the active transport of molecules across cell membranes.
- Signal transduction: ATP is involved in signaling pathways, acting as a substrate for kinases that phosphorylate proteins.
ATP in Metabolism
ATP is produced through various metabolic pathways, including:
- Glycolysis: A process that breaks down glucose to produce ATP.
- Citric acid cycle: Also known as the Krebs cycle, it generates ATP through the oxidation of acetyl-CoA.
- Oxidative phosphorylation: Occurs in the mitochondria, where ATP is produced from the electron transport chain.
ATP Binding and Protein Interaction
ATP binds to various proteins, often at specific motifs such as the Rossmann fold, which is a common structural motif in proteins that bind nucleotides like ATP.
Magnesium and ATP
Magnesium ions (Mg²⁺) are essential for the function of ATP. They stabilize the negative charges on the phosphate groups, facilitating the binding of ATP to enzymes and other proteins.
Related Pages
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD