Eschweiler–Clarke reaction
Eschweiler–Clarke Reaction
The Eschweiler–Clarke Reaction is a chemical reaction used primarily in organic chemistry for the methylation of primary and secondary amines. This reaction involves the reductive methylation of amines using formaldehyde and formic acid, resulting in the formation of tertiary amines or secondary amines, respectively. The reaction is named after the German chemists Wilhelm Eschweiler and Hans Thacher Clarke, who independently developed the method in the early 20th century.
Reaction Mechanism
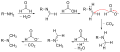
The Eschweiler–Clarke reaction proceeds through a series of steps. Initially, the amine reacts with formaldehyde to form an imine intermediate. Subsequently, this intermediate is reduced by formic acid, leading to the methylation of the nitrogen atom. In the case of primary amines, the process can repeat, allowing for the possibility of double methylation to form tertiary amines. The overall reaction can be summarized as follows:
- For primary amines: R-NH2 + 2 CH2O + 2 HCOOH → R-N(CH3)2 + 3 H2O
- For secondary amines: R2NH + CH2O + HCOOH → R2N-CH3 + 2 H2O
Applications
The Eschweiler–Clarke reaction is widely used in the synthesis of tertiary amines, which are important intermediates in the production of pharmaceuticals, agrochemicals, and dyes. This reaction is particularly valuable because it offers a straightforward method for the N-methylation of amines, a modification that can significantly alter the biological activity of a compound.
Advantages and Limitations
One of the main advantages of the Eschweiler–Clarke reaction is its simplicity and the use of readily available and relatively inexpensive reagents. However, the reaction has some limitations, including the potential for over-methylation and the use of formic acid, which can be hazardous in large quantities. Additionally, the reaction conditions may not be suitable for substrates that are sensitive to acidic environments.
Related Reactions
The Eschweiler–Clarke reaction is related to other reductive alkylation methods, such as the Leuckart Reaction, which also involves the use of formic acid but employs ammonium formate as the reducing agent. Another related reaction is the Bouveault-Blanc Reduction, which is used for the reduction of esters to primary alcohols.
See Also
References
- Eschweiler–Clarke reaction
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD