Structural isomer
Structural isomerism or constitutional isomerism is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders, as opposed to stereoisomerism. There are multiple forms of structural isomerism, including chain isomerism, functional group isomerism, tautomeric isomerism, positional isomerism, ring-chain isomerism, and functional isomerism.
Chain Isomerism
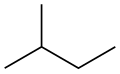
Chain isomerism, or n-isomerism, is a form of isomerism where the carbon atoms in a carbon chain are arranged in different ways. This form of isomerism is most commonly found in alkanes.
Functional Group Isomerism
Functional group isomerism occurs when one functional group is split up and distributed to make another functional group. This form of isomerism is common in organic compounds.
Tautomeric Isomerism
Tautomeric isomerism is a form of isomerism where the isomers are in equilibrium, and the equilibrium is fast enough that both isomers can be isolated separately. This form of isomerism is common in organic compounds.
Positional Isomerism
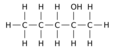
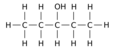
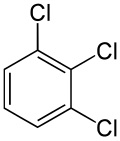
Positional isomerism is a form of isomerism where the functional group remains the same but the position of the functional group on the chain changes. This form of isomerism is common in organic compounds.
Ring-Chain Isomerism
Ring-chain isomerism is a form of isomerism where the carbon atoms can form either a ring or a chain. This form of isomerism is common in organic compounds.
Functional Isomerism
Functional isomerism is a form of isomerism where the molecular formula remains the same but the functional group changes. This form of isomerism is common in organic compounds.
See Also
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD