Ketene
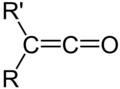
Ketene is an organic compound with the chemical formula C2H2O. It is a highly reactive ethylene derivative and is the simplest member of the ketenes, a group of compounds characterized by the functional group R2C=C=O, where R can be a variety of carbon-containing substituents. Ketenes are used in the production of acetic acid, acetic anhydride, and various acetyl derivatives, serving as important intermediates in organic synthesis and industrial chemistry.
Properties and Structure
Ketene is a colorless, poisonous gas with a pungent odor. It is extremely reactive due to the presence of a strained double bond between the carbon and oxygen atoms, making it an efficient acylating agent. The molecule consists of a planar arrangement of atoms, with the central carbon atom double bonded to both an oxygen atom and another carbon atom, forming a linear structure. This configuration contributes to its high reactivity, particularly in addition reactions with nucleophiles.
Synthesis
Ketene is typically synthesized through the pyrolysis of acetic acid or acetone. The process involves heating these substances to a high temperature, causing a decomposition reaction that produces ketene along with other byproducts. Another method involves the dehalogenation of dichloroethene in the presence of a strong base.
Reactions
Due to its high reactivity, ketene readily undergoes addition reactions with a variety of nucleophiles, including water, alcohols, and amines, to form carboxylic acids, esters, and amides, respectively. These reactions are often exploited in organic synthesis to introduce acyl groups into molecules.
Applications
Ketene's reactivity makes it a valuable intermediate in the chemical industry. It is used in the synthesis of acetic acid, one of the most important industrial chemicals, through its hydration. Ketene also reacts with alcohols to produce esters, which are important in the manufacture of plastics, solvents, and pharmaceuticals. Additionally, its reaction with amines yields amides, which are used in the production of plastics and rubber.
Safety
Ketene is highly toxic and can cause severe respiratory issues upon inhalation. It is also flammable, posing a risk of fire and explosion. Proper safety measures, including adequate ventilation and the use of protective equipment, are essential when handling ketene.
Transform your life with W8MD's budget GLP-1 injections from $125.
W8MD offers a medical weight loss program to lose weight in Philadelphia. Our physician-supervised medical weight loss provides:
- Most insurances accepted or discounted self-pay rates. We will obtain insurance prior authorizations if needed.
- Generic GLP1 weight loss injections from $125 for the starting dose.
- Also offer prescription weight loss medications including Phentermine, Qsymia, Diethylpropion, Contrave etc.
NYC weight loss doctor appointments
Start your NYC weight loss journey today at our NYC medical weight loss and Philadelphia medical weight loss clinics.
- Call 718-946-5500 to lose weight in NYC or for medical weight loss in Philadelphia 215-676-2334.
- Tags:NYC medical weight loss, Philadelphia lose weight Zepbound NYC, Budget GLP1 weight loss injections, Wegovy Philadelphia, Wegovy NYC, Philadelphia medical weight loss, Brookly weight loss and Wegovy NYC
|
WikiMD's Wellness Encyclopedia |
| Let Food Be Thy Medicine Medicine Thy Food - Hippocrates |
Medical Disclaimer: WikiMD is not a substitute for professional medical advice. The information on WikiMD is provided as an information resource only, may be incorrect, outdated or misleading, and is not to be used or relied on for any diagnostic or treatment purposes. Please consult your health care provider before making any healthcare decisions or for guidance about a specific medical condition. WikiMD expressly disclaims responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this site. By visiting this site you agree to the foregoing terms and conditions, which may from time to time be changed or supplemented by WikiMD. If you do not agree to the foregoing terms and conditions, you should not enter or use this site. See full disclaimer.
Credits:Most images are courtesy of Wikimedia commons, and templates, categories Wikipedia, licensed under CC BY SA or similar.
Contributors: Prab R. Tumpati, MD